Galvanic Corrosion: What You NEED to Know
The selection of gutter material must account for the potential of Galvanic or Electrolytic Corrosion at contact points with other metals. Special selection is also required for corrosive environments such as coastal or polluted industrial atmospheres.
Galvanic Corrosion can occur when dissimilar metals are in contact in the presence of an electrolyte. An electrolyte is any non-metal substance that will conduct an electric current.
Water, particularly rainwater, is a good electrolyte.
REDUCE GALVANIC CORROSION
- Avoid contact between metals that are father apart on the scale.
- Do not couple a fastener (FIG 2), with a large area of dissimilar metal.
- Coat the electronegative metal with a suitable paint or other non-metallic coating (or coat both surfaces where they meet).
- Separate the metals by tape, gasket, waterproof paper, elastomeric sheet, sealant or other non-absorbent, non-conductive material.
- Do not allow runoff from an electronegative metal to drain onto an electropositive metal, even if the metals are not in contact.
- Remove metal particles that are deposited from steel dies on formed metals.
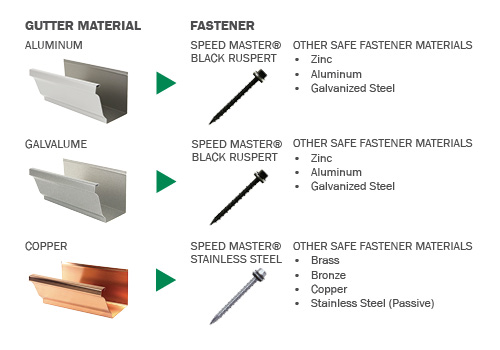
Fight Galvanic Corrosion with Speed Master Screws
Our Speed Master® Black Ruspert screws have been making aluminum and steel rain gutter installation easier for years, and now that same convenience and quality in stainless steel for high end copper jobs. Do the job right, with Speed Master® screws.
Speed Master® Screws at Senox.com
For Additional information See
ASTM Standard G82
ASTM STP 576
Wikipedia: Galvanic Corrosion

 Previous Post
Previous Post Next Post
Next Post